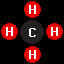
 methane
(CH4), the primary constituent of liquefied
or compressed natural gas, and
methane
(CH4), the primary constituent of liquefied
or compressed natural gas, and propane
(C3H8), the primary constituent of liquified
petroleum gas.
propane
(C3H8), the primary constituent of liquified
petroleum gas.Home - Background: Fuel Chemistry
This page shows the chemical structure of various alternative fuels, and discusses why the following aspects of different fuels have an effect on their tailpipe emissions:
Alternative fuels tend to be made up of small, fairly simple
molecules; for example, here are schematic chemical diagrams (C
denotes a carbon atom, H is hydrogen, and O is oxygen) of
 methane
(CH4), the primary constituent of liquefied
or compressed natural gas, and
methane
(CH4), the primary constituent of liquefied
or compressed natural gas, and
 propane
(C3H8), the primary constituent of liquified
petroleum gas.
propane
(C3H8), the primary constituent of liquified
petroleum gas.
Petroleum fuels are blends of lots of different chemical species;
in general, the molecules of a liquid petroleum fuel are pretty big
and complex. Here is
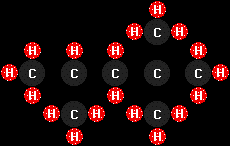 isooctane
(C8H18), typical of the molecules found in
gasoline (I had to spread out the structure a bit to get all the
hydrogen atoms to fit in the picture--all of these molecules are, of
course, three-dimensional, but some squish into a plane better than
others!), and
isooctane
(C8H18), typical of the molecules found in
gasoline (I had to spread out the structure a bit to get all the
hydrogen atoms to fit in the picture--all of these molecules are, of
course, three-dimensional, but some squish into a plane better than
others!), and

this monster is cetane, or n-hexadecane
(C16H34), typical of diesel fuel.
When a hydrocarbon fuel (that is, one that is made up of hydrogen and carbon) burns completely, the oxygen in the air combines with the hydrogen to form water (H2O) and with the carbon to form carbon dioxide (CO2). If the burning is not complete, then some of the carbon atoms only combine with one oxygen atom rather than two, to form carbon monoxide (CO), a highly poisonous gas.
Some of the carbon atoms may remain stuck together with each other and with some of the hydrogen atoms as well, so that unburned hydrocarbon molecules (mostly smaller than the ones in the original fuel) can also come out the tailpipe. These unburned hydrocarbons (plus any fuel hydrocarbons that evaporate from the fuel system before getting into the engine to be burned at all) react with nitrogen oxides (another pollutant from combustion) in the presence of sunlight to form ozone, which is a lung irritant (the "ozone layer" in the stratosphere is a shield against the sun's ultraviolet light, but at ground level ozone is the main component of "photochemical smog"). Carbon atoms can also remain stuck to one another with few or no hydrogen atoms attached, especially during incomplete combustion of diesel fuel, producing soot.
This is one of the reasons alternative fuels are less polluting than gasoline and diesel: their simpler molecules are easier to burn more completely in an engine, so that less carbon monoxide, soot, and unburned hydrocarbons come out the tailpipe. In addition, any unburned hydrocarbons that are produced are less reactive than those that come from incomplete burning of gasoline or diesel fuel, and so they produce less ground-level ozone; methane in particular is almost incapable of forming smog.
Some alternative fuels are not hydrocarbons; alcohols and
biodiesel contain oxygen atoms as well as
carbon and hydrogen. Here are the chemical structures of the common
alcohol fuels,
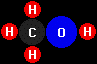 methanol
(CH3OH) and
methanol
(CH3OH) and
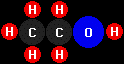 ethanol
(C2H5OH).
ethanol
(C2H5OH).
(Biodiesel molecules are "monoalkyl esters", but I haven't been able
to trace down anything more specific. The "ester" part of that name,
however, indicates that the molecules include oxygen atoms.)
In many parts of the USA, gasoline is "oxygenated" during at least part of the year; this means that oxygen-bearing compounds are added to the fuel mixture. The reason for doing this is that having some oxygen as part of the fuel molecules to start with promotes more complete combustion, so that less carbon monoxide, soot, and unburned hydrocarbons come out the tailpipe, as described above. Alcohol fuels and biodiesel carry this one step further, in that the oxygen-bearing compound is not an additive at the 5 to 10 percent level, but a major constituent of the fuel, which increases the benefits of oxygenation.
Even if, with the aid of electronic engine controls and efficient catalytic converters, a hydrocarbon fuel is burned completely to water and carbon dioxide, there is now growing concern about carbon dioxide as a greenhouse gas. Measures to cut back on production of carbon dioxide by automobiles without sacrificing performance can focus on efficiency, i.e., getting as much useful propulsive power out of a given amount of fuel as possible, which typically involves replacing the traditional drivetrain of a piston engine driving the wheels through a gearbox with a more efficient design.
However, some fuels inherently produce less carbon dioxide when burned completely than gasoline or diesel fuel. For example, counting the numbers of oxygen atoms it takes to burn up an isooctane molecule and a methane molecule (typical of gasoline and natural gas respectively), one can calculate that 100 oxygen atoms will combine with four isooctane molecules to produce 32 carbon dioxide molecules and 36 water molecules, while the same number of oxygen atoms will combine with 25 methane molecules to produce 25 carbon dioxide molecules and 50 water molecules. That is, a given amount of air (oxygen) will produce about 25% less carbon dioxide if used to burn natural gas than if used to burn gasoline. (Of course, this advantage will be reduced if you have to open the throttle wider and burn an additional amount of air with natural gas to get the same amount of power, but in the real world the 25% figure turns out to be about right.)
The other thing to consider is the source of the carbon in the fuel; if it came from the carbon dioxide in today's air to begin with, like an alcohol fuel produced by fermenting biomass (as opposed to a fossil fuel, whose carbon came out of the air when the dinosaurs were around!), then returning it to the air now adds nothing to the net flow of carbon dioxide into the atmosphere. Alcohol fuels or biodiesel produced from plants, when burned, just return to the air the carbon dioxide that those plants took out of the air while growing.
Finally, there's one fuel that, in itself, produces no carbon
dioxide at all when burned, namely
![]() hydrogen;
there's no carbon there to produce carbon dioxide!
hydrogen;
there's no carbon there to produce carbon dioxide!
Of course, since free hydrogen molecules don't occur in nature, it is
typically produced by "reforming" a hydrocarbon or alcohol fuel or by
using electricity to split water into hydrogen and oxygen. Then the
size of the contribution of hydrogen fuel to carbon dioxide emissions
depends on the source of the hydrocarbon fuel that was reformed or
the source of the electricity used to split the water.
If a fossil fuel was the ultimate source of the energy that is, in effect, stored in the hydrogen, then you can still gain a large improvement in carbon-dioxide production if the hydrogen is used in an efficient drivetrain, as noted above; the same is true for the electrical energy stored in a battery-powered electric vehicle. In order to obtain the full benefits of reduction of carbon dioxide (or of ordinary air pollutants like carbon monoxide), of course, the energy used to split the hydrogen or charge the battery can be obtained from a renewable source like wind power or photovoltaics.
The nice thing about hydrogen- or battery-powered vehicles is that they can run on whatever is available--efficient natural-gas-burning powerplants today, with an increasing contribution from renewable energy as time goes on and the price of photovoltaic cells (solar cells) and other renewable energy sources continues to decline. As renewable energy becomes an ever larger part of the power generation mix over the next few decades, hydrogen- and battery-powered vehicles can switch over to the new power sources without a hiccup--it's all electricity to them!
 Comparison
of Alternative Fuels
Comparison
of Alternative Fuels Back
to Fueling Station
Back
to Fueling Station Site
Map
Site
Map Contact
Me
Contact
Me All content copyright 1998-2024 by Mark Looper, except as noted. Reuse of my copyrighted material is authorized under Creative Commons Attribution 4.0 International license (CC BY 4.0).
![]()
![]()
new 11 July 1998